Pioneering Innovation
Our R&D department is at the forefront of discovering and developing new drugs and treatments to address various medical conditions. Our team comprises scientists, researchers, clinicians, and specialists with expertise in chemistry, biology, pharmacology, and related fields.
We have our own R&D specialized team with years of experience so we can provide you with the utmost efficacious products.


This involves identifying potential drug targets, screening compounds for their therapeutic effects, and optimizing lead candidates. The process often involves a combination of computational biology, high-throughput screening, and medicinal chemistry to develop new drugs that can effectively treat various diseases.
Our research team carefully evaluates hundreds of chemical compounds to identify the most promising candidates for further development and clinical testing.
Once a promising candidate is identified, preclinical testing is conducted using laboratory and animal models. This phase evaluates the drug's safety profile, potential side effects, and preliminary efficacy data. The goal is to gather sufficient evidence to support an Investigational New Drug (IND) application.
Our specialized laboratories employ advanced techniques to thoroughly test compounds before they move forward to human trials.
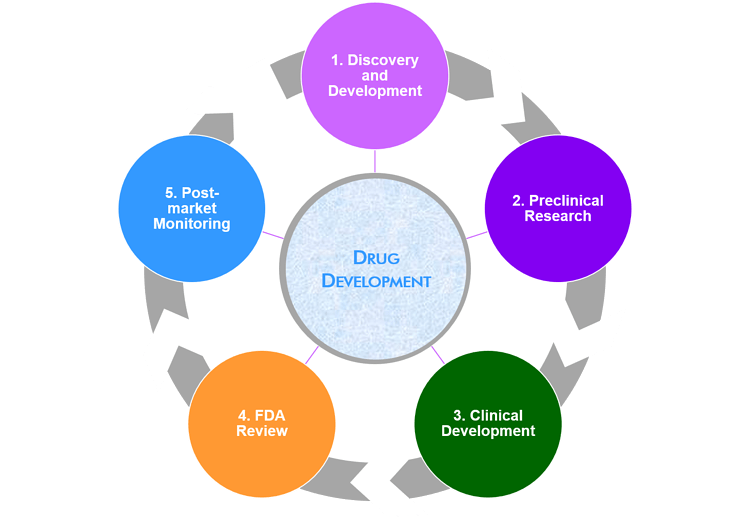
This involves rigorous testing and evaluation of the drug in human subjects across multiple phases. Phase I focuses on safety and dosage, Phase II evaluates efficacy and side effects, Phase III compares the new drug with existing treatments, and Phase IV involves post-marketing surveillance. Our clinical teams work closely with hospitals and medical institutions worldwide.
We conduct thorough and transparent clinical trials to ensure high-throughput screening and validation.
Our regulatory affairs team manages the submission of documentation and data to regulatory agencies such as the FDA. They ensure compliance with all applicable regulations and work to obtain marketing approval for new drugs. This process involves preparing comprehensive regulatory dossiers that support the safety and efficacy of the drug.
We maintain rigorous compliance with all regulatory requirements across different jurisdictions and therapeutic areas.

This bridges the gap between basic research and clinical application. Translational medicine focuses on applying laboratory discoveries to develop practical applications and new approaches to diagnosis and treatment. Our team collaborates to accelerate the development of promising research findings into viable therapeutic options.
Our commitment to translational research ensures seamless progression from concept to clinical practice.
We collaborate with leading academic institutions, research organizations, and healthcare providers worldwide. These partnerships accelerate innovation and provide access to cutting-edge research facilities and expertise. Our collaborative approach ensures we deliver breakthrough treatments that address unmet medical needs.
Through strategic partnerships, we expand our research capabilities and enhance our ability to bring new medicines to market.
Our commitment to continuous improvement ensures we stay at the forefront of pharmaceutical innovation. We invest in training and development for our research team, stay updated with the latest scientific advancements, and adapt our strategies to incorporate new technologies and methodologies.
We recognize that innovation requires dedication, continuous learning, and the courage to pursue bold ideas that may ultimately transform patient care.
If you think it's just you're looking for, Please contact us.
Call Us : +91-7018754076
Contact Us© 2025 . All Rights Reserved by Buzingbee.com